A Decade of 18.65% CAGR Growth Ahead for AI in Regulatory Affairs Ecosystems
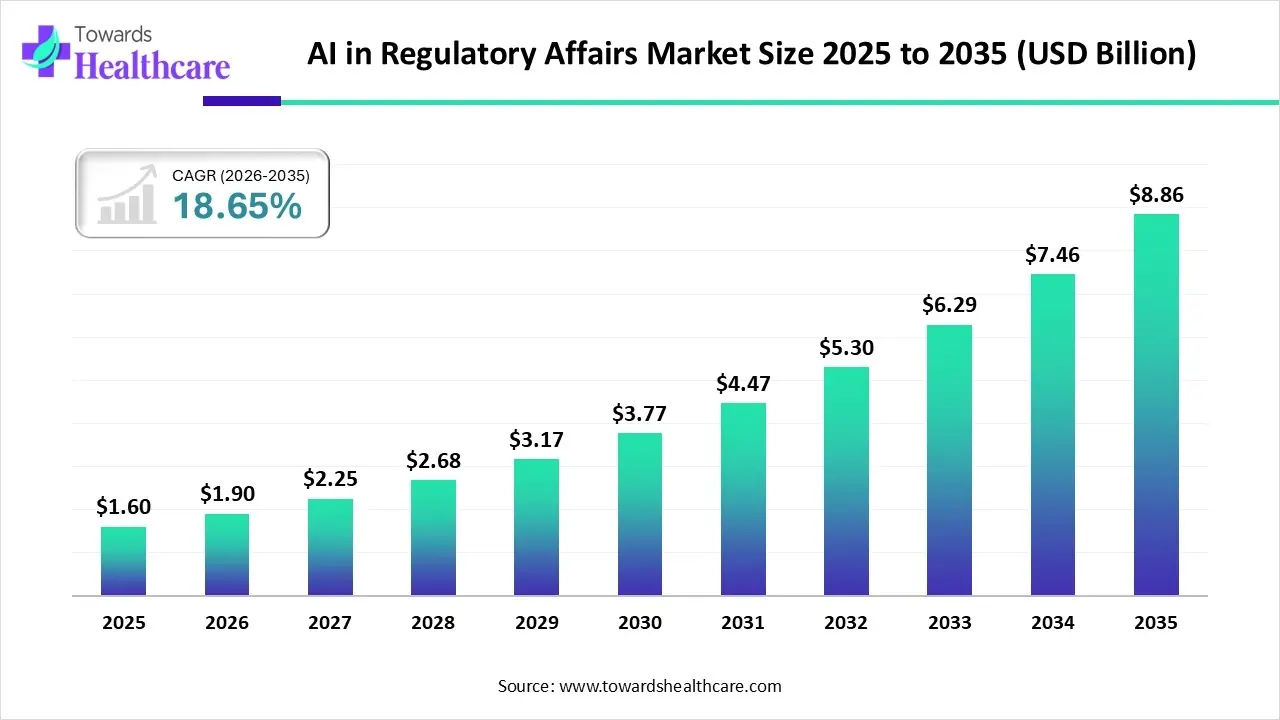
The global AI in regulatory affairs market size was valued at USD 1.6 billion in 2025 and is predicted to hit around USD 8.86 billion by 2035, rising at a 18.65% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
Ottawa, Feb. 17, 2026 (GLOBE NEWSWIRE) -- The global AI in regulatory affairs market size is calculated at USD 1.9 billion in 2026 and is expected to reach around USD 8.86 billion by 2035, growing at a CAGR of 18.65% for the forecasted period.
Get a customized sample pages highlighting AI in global regulatory affairs | Download Now @ https://www.towardshealthcare.com/download-sample/6466
Key Takeaways
- The AI in regulatory affairs market will likely exceed USD 1.6 billion by 2025.
- Valuation is projected to hit USD 8.86 billion by 2035.
- Estimated to grow at a CAGR of 18.65% starting from 2026 to 2035.
- North America dominated the market in 2024.
- Asia Pacific is expected to witness rapid expansion during the forecast period.
- By component, the software/platforms segment registered dominance in the market in 2024.
- By component, the services segment is expected to be the fastest-growing in the coming years.
- By deployment mode, the cloud-based segment led the market in 2024 and is expected to grow rapidly during 2025-2034.
- By application, the regulatory intelligence segment was dominant in the market in 2024.
- By application, the pharmacovigilance & safety reporting segment is expected to witness the fastest growth in the studied years.
- By end-use, the pharmaceutical companies segment registered dominance in the AI in regulatory affairs market in 2024.
- By end-use, the CRO/CDMO segment is expected to be the fastest-growing in the upcoming years.
How is the AI Assisting Regulatory Affairs Across the Globe?
The use of machine learning, natural language processing (NLP), & automation in simplifying compliance, document generation, and submission processes for industries, such as pharmaceuticals & medical devices, describes the AI in regulatory affairs market. Moreover, AI algorithms are supporting submission management through streamlining eCTD 4.0 submissions & allowing for automated document tagging and module compilation. Alongside, AI has a major role in escalating safety monitoring by scanning literature, social media, and regulatory databases for adverse events, and is encouraging robust risk-benefit assessments.
What are the Key Drivers in the AI in Regulatory Affairs Market?
Ongoing heavy investments in R&D and surging competition are driving companies to adopt AI for lowering manual labor in document generation, alleviating submission preparation time by 20–30%. Besides this, the emergence of integrated Gen AI, Natural Language Processing (NLP), & machine learning is supporting excellent data analysis, predictive modeling of approval timelines, & automation of routine tasks.
We’re happy to help with your order or any questions connect with us at sales@towardshealthcare.com
What are the Major Drifts in the AI in Regulatory Affairs Market?
- In October 2025, Peer AI raised $12.1 million in total funding to speed up drug approvals with an intelligent regulatory workflow.
- In October 2025, Parexel partnered with Weave Bio, focused on boosting regulatory submission processes and the time to market for novel therapies.
-
In September 2025, IBM & BharatGen joined to broaden the adoption of Artificial Intelligence (AI) in India, led by BharatGen’s sovereign multimodal and Large Language Models (LLMs).
What is the Emerging Challenge in the AI in Regulatory Affairs Market?
A major limitation is the consistent upgradation of regulatory guidelines, which results in uncertainty for companies in executing advanced technologies. The global adoption of AI demands significant investment in technology, infrastructure, and training, which can lead to inconsistency, especially for smaller players.
Regional Analysis
How did North America Dominate the Market in 2024?
In 2024, North America held the biggest share of the AI in regulatory affairs market, due to the favourable FDA initiatives, greater investment in health tech, & regulatory intelligence. Recently, the FDA published its first draft guidance called "Considerations for the Use of Artificial Intelligence to Support Regulatory Decision-Making", which emphasizes transparency, validation, and lifecycle management of AI tools in drug development & submissions.
AI adoption in U.S. regulatory affairs has accelerated with advanced technological infrastructure, a life sciences focus, and agencies like the FDA deploying generative tools for scientific reviews and compliance tasks. Firms increasingly use AI for submissions, monitoring, and regulatory intelligence.
For instance,
- In June 2025, the U.S. Food and Drug Administration (FDA) unveiled Elsa, a generative Artificial Intelligence (AI) tool to assist employees from scientific reviewers to investigators in working more effectively.
Why did the Asia Pacific Expand Significantly in the Market in 2024?
During the prospective period, the Asia Pacific is estimated to witness rapid growth in the AI in regulatory affairs market. The regional progression is mainly propelled by surging biotech innovations and AI assistance in handling complex regulatory filings, which enables expedited approvals by following various regional regulations. Whereas China is aiming at targeted, risk-based rules and industry-specific standards. Alongside, Vietnam and other Southeast Asian nations are increasingly leveraging risk-based AI laws, & India is promoting itself as a leader in AI governance for the Global South.
In China, AI integration in regulatory processes is expanding as government and industry explore automation for document handling, compliance monitoring, and regulatory data analysis, supported by state-led policies promoting “AI+” applications across sectors, though regulatory frameworks are still evolving to govern safe, standardized use.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Segmental Insights
By component analysis
Which Component Led the AI in Regulatory Affairs Market in 2024?
The software/platforms segment captured the dominating share of the market in 2024. This mainly comprises cloud-based solutions integrated with Regulatory Information Management (RIM) systems to simplify workflows. The latest advanced platforms, such as RegDesk, RegASK, & DDReg Pharma, are accelerating scanning of global databases. Novel platforms are allocating digital workers or AI agents to handle complex, multi-step workflows, including end-to-end dossier compilation, labeling updates, & submission.
Moreover, the services segment is estimated to expand rapidly during the predicted timeframe. AI breakthroughs are exploring automated document generation, compliance monitoring, and AI-enabled regulatory intelligence. Extensive generative AI is developing and reviewing regulatory documents, especially clinical study reports, investigator brochures, & dossiers, which simplifies submission processes. Emerging rigorous services encompass the analysis of images & conversion of unstructured data, such as scanned PDFs, into actionable insights.
By deployment mode analysis
Why did the Cloud-based Segment Dominate the Market in 2024?
In 2024, the cloud-based segment held a major share and is expected to grow at the fastest rate during the forecast period. The segmental expansion is fueled by the growing need for raised scalability, affordable infrastructure, and real-time alliance in pharmaceutical & medical device submissions. Also, these solutions are facilitating strong, built-in security features, such as data encryption and continuous monitoring, which are vital for sensitive regulatory data.
By application analysis
Which Application Led the AI in Regulatory Affairs Market in 2024?
The regulatory intelligence segment held a dominant share of the market in 2024. The globe is widely using machine learning (ML) & natural language processing (NLP) to automate surveillance, interpretation, and mapping of global regulatory modifications. Whereas, AI systems are consistently tracking and analyzing thousands of regulatory agency websites, scientific publications, & patent filings to find changes.
However, the pharmacovigilance & safety reporting segment will show rapid growth. Rising need to speed up PV tasks, including individual case safety report (ICSR) processing and literature screening, lowering the time from adverse event (AE) detection to regulatory submission, is strengthening the use of AI solutions. Alongside, the FDA-like regulatory bodies are also fostering integration of AI in PV to shift from passive reporting to active, near real-time safety surveillance.
By end-use analysis
How did the Pharmaceutical Companies Segment Dominate the Market in 2024?
In 2024, the pharmaceutical companies segment was dominant in the market. Several giant firms, like Sanofi, Pfizer, and Novartis, are highly leveraging AI in regulatory affairs to automate dossier management, bolster compliance, and speed up time-to-market for drugs. Nowadays, they are pushing AI platforms to assess historical health authority queries and global regulatory trends to estimate approval risks, which allows proactive compliance.
However, the CRO/CDMO segment is anticipated to expand fastest. The emergence of AI in CDMOs is assisting in the prediction of CMC (Chemistry, Manufacturing, and Controls) risks, estimating supply chain challenges, & improving manufacturing quality monitoring, often fueled by the need for onshoring in response to legislation. Currently, CRO/CDMOs are using AI-assisted solutions to simplify eCTD submissions, automate data coding, & manage complex, decentralized trial documentation.
Grow your business with our research expertise | Let’s Collaborate https://www.towardshealthcare.com/schedule-meeting
What are the Significant Developments in the AI in Regulatory Affairs Market?
- In February 2026, Global Key Solutions Corp. launched KeyPedia, an AI-driven regulatory intelligence platform purpose-built for pharmaceutical quality operations.
- In January 2026, EDETEK Inc. unveiled Ensemble, a comprehensive AI Managed Services to support sponsors in operationalizing validated, human-supervised AI across clinical development.
-
In May 2025, Basil Systems introduced Insights, a novel feature within its Basil Intel for Pharma platform, which raises regulatory compliance and planned decision-making.
Key Players List

- IQVIA
- Freyr Solutions
- DDReg Pharma
- RegASK
- Deloitte (via its RegAI solution)
- IBM
- Oracle
- Microsoft
- Google (Alphabet)
- Tempus AI
- Accenture
- Wipro
- Zenovel
- Innoplexus
- Workiva
- ComplyAdvantage
- MetricStream
- Viz.ai
- ArisGlobal
- Veeva Systems
Browse More Insights of Towards Healthcare:
The global regulatory affairs market size is calculated at US$ 16 billion in 2024, grew to US$ 17.37 billion in 2025, and is projected to reach around US$ 36.33 billion by 2034. The market is expanding at a CAGR of 8.55% between 2025 and 2034.
The Europe clinical trials market size is calculated at US$ 11.04 billion in 2025, grew to US$ 11.65 billion in 2026, and is projected to reach around US$ 18.81 billion by 2035. The market is expanding at a CAGR of 4.80% between 2026 and 2035.
The lipid regulators market size stood at US$ 33.65 billion in 2025, grew to US$ 35.01 billion in 2026, and is forecast to reach US$ 49.96 billion by 2035, expanding at a CAGR of 4.04% from 2026 to 2035.
The epilepsy drugs market size is projected to reach USD 16.42 billion by 2034, growing from USD 10.49 billion in 2025, at a CAGR of 5.1% during the forecast period from 2025 to 2034.
The ulcerative colitis treatment market is anticipated to grow from USD 11.01 billion in 2025 to USD 15.81 billion by 2034, with a compound annual growth rate (CAGR) of 4.1% during the forecast period from 2025 to 2034.
The global anti-inflammatory drugs market size is calculated at US$ 122.32 in 2024, grew to US$ 132.63 billion in 2025, and is projected to reach around US$ 274.79 billion by 2034. The market is projected to expand at a compound annual growth rate (CAGR) of 8.43% between 2025 and 2034.
The global medical affairs outsourcing market size is calculated at USD 2.55 billion in 2025, grew to USD 2.90 billion in 2026, and is projected to reach around USD 9.16 billion by 2035. The market is expanding at a CAGR of 13.64% between 2026 and 2035.
The filtration in biologics market size stood at US$ 8.44 billion in 2025, grew to US$ 9.55 billion in 2026, and is forecast to reach US$ 29.01 billion by 2035, expanding at a CAGR of 13.14% from 2026 to 2035.
The global bone marrow transplant market size is calculated at US$ 10.75 in 2024, grew to US$ 11.24 billion in 2025, and is projected to reach around US$ 16.76 billion by 2034. The market is expanding at a CAGR of 4.54% between 2025 and 2034.
The global bio burden testing market size is calculated at US$ 1.77 in billion 2025, grew to US$ 2.03 billion in 2026, and is projected to reach around US$ 6.82 billion by 2035. The market is expanding at a CAGR of 14.44% between 2026 and 2035.
Segments Covered in the Report
By Component
- Software/Platforms
- Services
By Deployment Mode
- Cloud-based
- On-Premises
By Application
- Regulatory Intelligence
- Data Migration & Integration
- Dossier Management
- Document Management
- Product Registration & Approvals
- Pharmacovigilance & Safety Reporting
- Regulatory Submissions & Publishing
- Others
By End-use
- Pharmaceutical Companies
- Biotechnology Companies
- Medical Device Companies
- CRO/CDMO
- Others
By Region
-
- North America
- U.S.
- Canada
- Mexico
- Rest of North America
- South America
- Brazil
- Argentina
- Rest of South America
- Europe
- Western Europe
- Germany
- Italy
- France
- Netherlands
- Spain
- Portugal
- Belgium
- Ireland
- UK
- Iceland
- Switzerland
- Poland
- Rest of Western Europe
- Eastern Europe
- Austria
- Russia & Belarus
- Türkiye
- Albania
- Rest of Eastern Europe
- Western Europe
- Asia Pacific
- China
- Taiwan
- India
- Japan
- Australia and New Zealand,
- ASEAN Countries (Singapore, Malaysia)
- South Korea
- Rest of APAC
- MEA
- GCC Countries
- Saudi Arabia
- United Arab Emirates (UAE)
- Qatar
- Kuwait
- Oman
- Bahrain
- South Africa
- Egypt
- Rest of MEA
- GCC Countries
- North America
Secure your premium report and explore detailed analysis | Buy Now @ https://www.towardshealthcare.com/checkout/6466
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Towards Packaging | Towards Food and Beverages | Towards Chemical and Materials | Towards Dental | Towards EV Solutions | Healthcare Webwire
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest
Explore more:
https://www.towardshealthcare.com/insights/ai-in-life-science-market
https://www.towardshealthcare.com/insights/ai-in-biotechnology-market-sizing
https://www.towardshealthcare.com/insights/ai-in-ophthalmology-market-sizing
https://www.towardshealthcare.com/insights/ai-in-medical-scheduling-software-market-sizing
https://www.towardshealthcare.com/insights/ai-in-biopharmaceuticals-market-sizing
https://www.towardshealthcare.com/insights/healthcare-cloud-computing-market-sizing
https://www.towardshealthcare.com/insights/metaverse-in-healthcare-market-size
https://www.towardshealthcare.com/insights/laboratory-information-management-systems-market-sizing
https://www.towardshealthcare.com/insights/pharmacy-automation-market-sizing

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

